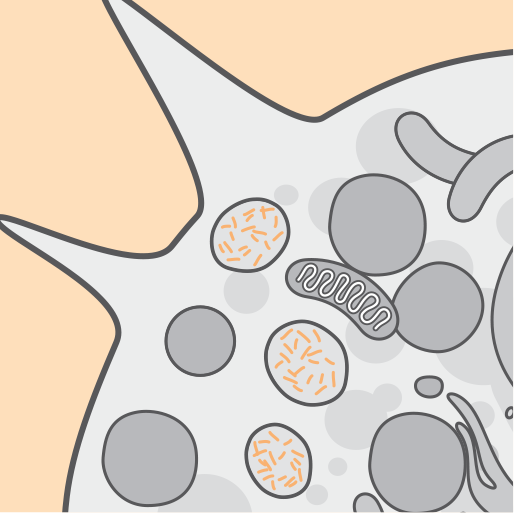
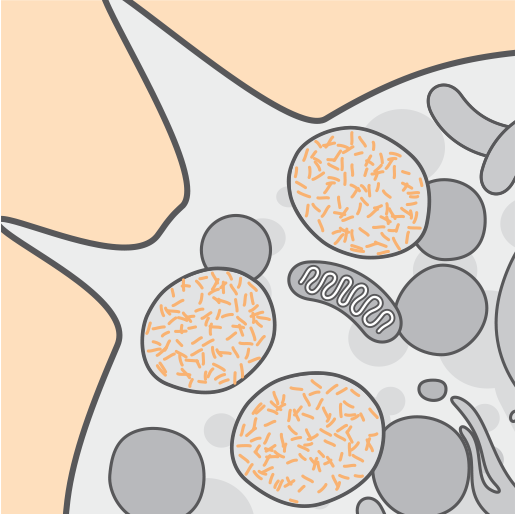
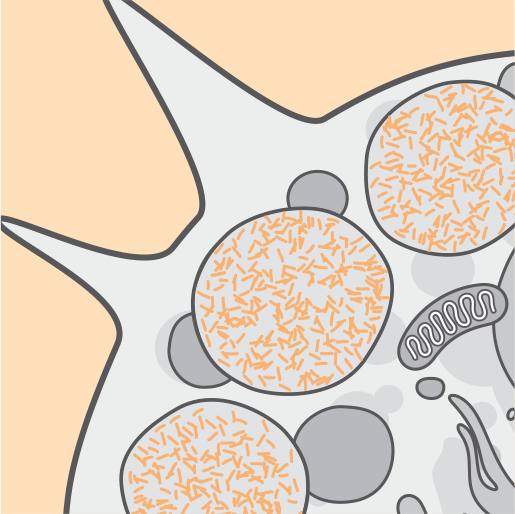
Pathophysiology
References: 1. Schulz A et al. NCL diseases — clinical perspectives. Biochim Biophys Acta 2013;1832:1801–1806. 2. Chang M et al. CLN2. In: Mole S, Williams R, and Goebel H, eds. The neuronal ceroid lipofuscinoses (Batten Disease). 2nd ed. Oxford, United Kingdom:Oxford University Press; 2011:80–109. 3. Mole SE, Williams RE. Neuronal ceroid-lipofuscinoses. In: Pagon RA, Adam MP, Ardinger HH, et al., eds. GeneReview® [internet]. Seattle, WA: University of Washington;1993–2016. 4. Mole SE et al. Correlations between genotype, ultrastructural morphology and clinical phenotype in the neuronal ceroid lipofuscinoses. Neurogenetics 2005;6:107–126. 5. Fietz M et al. Diagnosis of neuronal ceroid lipofuscinosis type 2 (CLN2 disease): Expert recommendations for early detection and laboratory diagnosis. Mol Genet Metab. 2016;119:160–167. 6. Haltia M. The neuronal ceroid-lipofuscinoses: From past to present. Biochim Biophys Acta 2006;1762:850–856.
 UK (English)
UK (English)
 Español
Español Türkçe
Türkçe Deutsch
Deutsch Italiano
Italiano Русский
Русский Français
Français